If antibodies are not credible, what is the point of science?
- Hao Shi
- Aug 7, 2025
- 3 min read
Updated: Aug 13, 2025
In biomedical research, antibody specificity and consistency form the cornerstone of experimental reproducibility. Over the past decades, scientists have injected proteins into animals (such as rabbits) to stimulate their immune systems to produce antibodies targeting specific protein signaling molecules. To achieve long-term and stable antibody supply, researchers have also fused animal immune cells with immortalized tumor cells to create hybridoma cell lines capable of antibody secretion. In the 1990s, as biotech companies began mass-producing antibodies, most researchers turned to direct procurement from commercial catalogs. Today, nearly 350 global suppliers collectively provide approximately 7.7 million research-grade antibody products. As an indispensable tool in scientific research, the specificity and quality stability of antibodies remain critical.
In a recent study in Nature, YCharOS (an organization that validates antibodies) tested 16 commercial antibodies marketed for recognizing the C9ORF72 protein. Only three met the criteria – a pass rate of less than 20%. Alarmingly, these ineffective antibodies have been cited over 3,000 times in 15 research papers. This isn't an isolated case! The persistent issue of low-efficiency antibodies has plagued the biomedical field, leading to resource waste and even being recognized as a contributing factor to the scientific reproducibility crisis – a pressing reality that demands urgent attention.
To address antibody specificity challenges, the International Working Group for Antibody Validation (IWGAV) has established five antibody characterization strategies: orthogonal strategies, independent antibody strategies, genetic strategies, recombinant expression strategies, and capture ms by mass spectrometry analysis (Figure 1). While each approach has its strengths and weaknesses, the gene editing strategy emerges as the gold standard for specificity validation. This method utilizes gene editing or RNA interference to knock out (KO) or knock down (KD) target protein signaling molecules. However, due to high validation costs and lengthy timelines, few antibody manufacturers currently adopt this approach.

Against this backdrop, YCharOS collaborates with commercial companies to systematically validate the specificity of commercially available antibodies. Their "knockout cell line control method" evaluates antibody performance by comparing antibody performance in wild-type normal cell lines versus those with knockout of target protein signaling molecules. To date, the project has tested over 1,000 antibodies targeting more than 100 human proteins. However, given the approximately 200,000 complex antibodies currently on the market, comprehensive validation remains a significant challenge. Developing rapid and cost-effective antibody specificity verification methods has become an urgent bottleneck requiring breakthroughs in the industry.
To address this challenge, GenuIN biotech has developed its proprietary ShGE™ gene silencing platform for high throughput validation of antibodies. By leveraging lentivirus-mediated shRNA interference technology, the company achieves efficient mRNA-level silencing of target genes. Combined with multi-applicational validation systems, these advancements significantly enhance quality improvements in antibody production. This breakthrough helps overcome the persistent issues of "low specificity and poor reproducibility" in both research and industrial applications. The unique advantages of GenuIN biotech's ShGE™ platform include:
1. Efficient gene silencing: The potential to cover nearly all ~20,000 human protein coding genes. The success rate of silencing increased from 40% of traditional technology to>70%. The cycle shortened from 6 months to 2 weeks. And the cost reduced by 95%.
2. Multi-dimensional verification system: Each antibodycan be tested by immunoblot analysis (Western blot analysis, WB), flow cytometry (flow cytometry, FCM) and immunocytochemical staining (immunocytochemical staining, IC) on gene-silenced cells. Thedatais open and traceable (Figure 2-4).
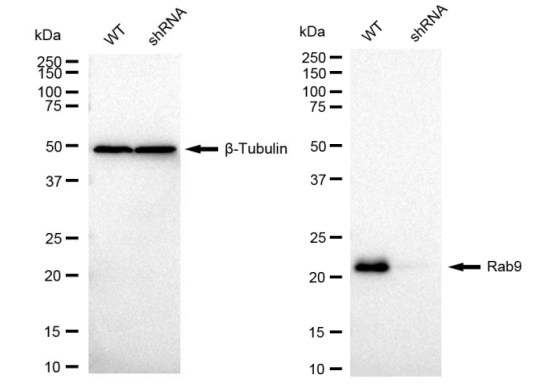


3. High-throughput validtion: In two years, GenuIN biotech has successfully constructed more than 1100 KD cell lines and more than 2000 corresponding monoclonal antibodies.
GenuIN biotech is committed to “solving antibody non-specificity challenges through genetic engineering methods.” We continuously develop and screen for high-quality, specific antibodies, ensuring every product undergoes validation through Western blotting (WB), flow cytometry, and immunocytochemistry.
references
1. Kwon D.Kwon D. The antibodies don't work! The race to rid labs of molecules that ruin experiments. Nature. 2024; 635(8037): 26-28. doi:10.1038/d41586-024-03590-0
2. Uhlen M, Bandrowski A, Carr S, Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nat Methods. 2016; 13(10): 823-827. doi:10.1038/nmeth.3995
08/08/2025
Long Hou
Deputy Director of R&D
GenuIN Biotechnologies


Comments